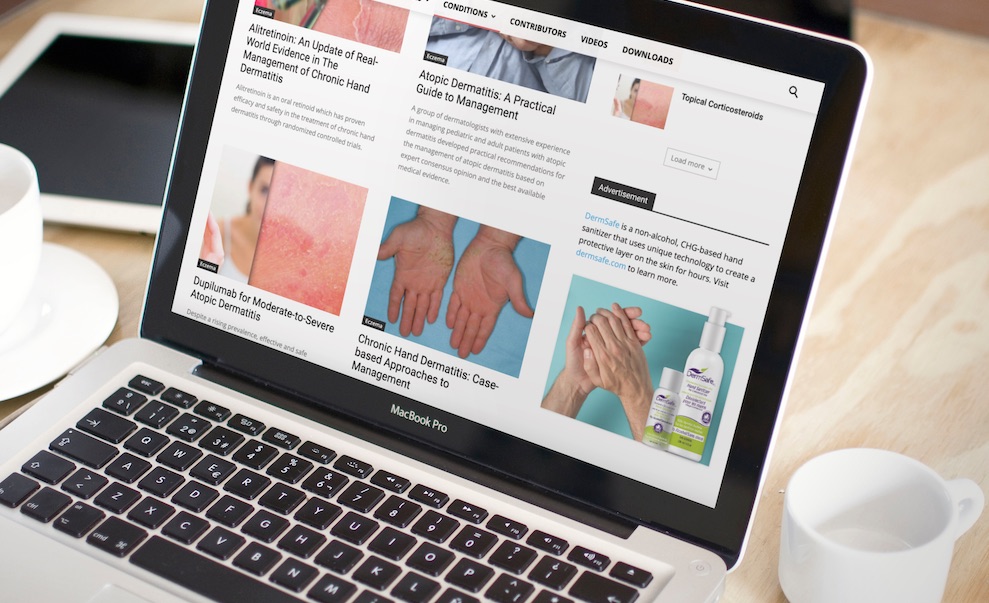
Ovation Science Accesses Canadian Consumers with Launch of Online Marketing Campaign for DermSafe Hand Sanitizer
Vancouver, BC – April 27, 2021 (CSE: OVAT and OTC: OVATF) – Ovation Science Inc. (“Ovation” or the “Company”) announces it has begun an online campaign to promote its DermSafe® hand sanitizer made without alcohol and powered by the Company’s Invisicare® drug delivery technology. The campaign will be featured on the Canadian Skin Information’s two key websites which attract over 25 thousand visitors per month. The aim of the campaign is to introduce the unique benefits of DermSafe to consumers looking for skin related information, to emphasize the importance of protecting your hands from all kinds of germs and to provide consumers with an easy access point to purchase DermSafe
“We are excited to increase our exposure of DermSafe through Skin Information’s online medical channels,” said Terry Howlett, President of Ovation. “DermSafe will be featured “in content” in over 100 articles through-out their two main websites. For example, since DermSafe is made without alcohol and has the added benefit of our proprietary polymer delivery system Invisicare®, it does not dry out your hands like alcohol hand sanitizers do. The ads are therefore being run in articles that address conditions like dry skin, eczema, contact dermatitis and other dry skin conditions.” He added,” We believe this online exposure will help sales of DermSafe in Canada while most of the country is on lockdown and cannot access retail locations. We are all waiting with great anticipation for the country to open back up and have everyone going back to work, back to sports and back to school.”
DermSafe is a game-changer in the hand sanitizer market. It is a pharmaceutical-grade hand sanitizer lotion that instead of alcohol, uses chlorhexidine gluconate to kill germs effectively; an ingredient used worldwide in hospital soaps as it has a proven ability to kill gram-negative and gram-positive bacteria as well as envelope viruses. COVID-19 is an envelope virus. The Company previously announced DermSafe was successfully tested against the human coronavirus (Surrogate to SARS-CoV-2) and these independent test results showed a 99.97% reduction in the virus. The Company also previously announced that DermSafe is also a recipient of the Canadian Dermatology Review Panel’s “Seal of Approval”.
The Skin Information Network of websites consists of a variety of consumer and medical websites with Skin Therapy Letter and Derm Letter being the key websites for complete dermatological information. Skin Therapy Letter is a prestigious, peer-reviewed publication that is indexed with the National Library in Washington, DC with articles being featured on PubMed and MEDLINE. Derm Letter is a skincare focused e-zine, delivering the latest news on products and treatments of various skin conditions, skincare products and procedures, to following the latest breakthrough studies in skin science. Examples where DermSafe ads are featured includes:
- https://www.skintherapyletter.com/eczema/
- https://dermletter.com/skin-conditions/irritant-contact-dermatitis/
For information about DermSafe and how to order visit https://dermsafe.com
For information about Ovation Science visit https://ovationscience.com.
Statements have not been evaluated by the Food and Drug Administration or Health Canada. These products are not intended to diagnose, treat, cure, or prevent any disease.
About Ovation Science Inc.
Ovation Science Inc. is a research and development company that develops topical and transdermal consumer products including its CBD/THC cannabis formulations including ARLO CBD Beauty and InVibe® MD (“health & wellness” line), and secondly, its unique DermSafe® hand sanitizer; all made with its patented Invisicare® skin delivery technology. The technology enhances the delivery of ingredients to and through the skin and is protected by patents in eleven countries. With over twenty years of topical and transdermal drug delivery experience in the pharmaceutical market, Ovation’s management and science team have created a unique pipeline of over twenty-five patent-protected medical / wellness topical and transdermal products along with a line of anti-aging / beauty formulas. Ovation earns revenues from licensing and development fees, royalties, the sale of Invisicare to its licensees along with revenue from its own product sales. Ovation has offices in Vancouver, BC Canada and Las Vegas, Nevada, USA. Ovation trades on the CSE under the symbol OVAT and in the USA on OTC Markets under the symbol OVATF. Visit our website www.ovationscience.com for more information.
Forward-Looking Statements
Information set forth in this news release contains forward-looking statements that are based on assumptions as of the date of this news release. These statements reflect management’s current estimates, beliefs, intentions and expectations and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. In particular there is no assurance that the Company’s DermSafe product will have increased online sales, and that any additional exposure will result in sales. Although DermSafe has been tested against other envelope viruses there is no assurance that it will kill or be as effective against COVID-19. In addition, there is no assurance the Company’s level of sales will continue or will not be negatively impacted by increased competition and recovery from the coronavirus pandemic. Other examples of the assumptions underlying the forward-looking statements contained herein include, but are not limited to those related to: strategies, potential sales, distribution and manufacturing of the Company’s product as well as its effectiveness against COVID-19, the Company’s ability to receive regulatory approval outside of Canada. There are no guarantees of future performance. Ovation Science Inc. cautions that all forward looking statements are inherently uncertain and that actual results may be affected by a number of material factors, many of which are beyond Ovation Science Inc.’s control. Accordingly, readers should not place undue reliance on the forward-looking information. Ovation disclaims any obligation to revise or update any such forward-looking information to reflect future results, events or circumstances, except as required by law.
Statements have not been evaluated by the Food and Drug Administration or Health Canada. These products are not intended to diagnose, treat, cure, or prevent any disease. Ovation does not sell or distribute any products that are in violation of the United States Controlled Substances Act (US.CSA).
Neither the Canadian Securities Exchange nor its Regulation Services Provider accepts responsibility for the adequacy or accuracy of this release.
Contact:
FOR INVESTOR RELATIONS:
Sebastian Kunyz:
ovat@kincommunications.com
Phone: 604-684-6730 or Toll Free at 866-684-6730
FOR BUSINESS DEVELOPMENT & CORPORATE INQUIRIES:
Doreen McMorran:
doreen@ovationscience.com
Phone: 604.283.0903 ext. 4